lattice energy of kf
Note that the data given has been perturbed so looking up the answer is. Web The lattice energies of KFKClKBr and KI follows the order.
 |
| Solved The Lattice Energy Of Kf Is 794 Kj Mol And The Interionic Distance Is 269 Pm The Na F Distance In Naf Which Has The Same Structure As Kf Is 231 Pm Which Of |
1 M a L b s a M b g b X a g This.

. The NaF distance in NaF which has the same structure as KF is 231 pm. Web How is lattice energy estimated using Born-Haber cycle. Web Lattice energy depends on. The estimated lattice energy for is CsF22347kjmol.
17 Dislike Share Save. The lattice energy. Web The lattice energy of KF. Web 816 rows Equivalently lattice energy can be defined as the amount of work energy that is released.
Web The lattice energy of KF is 794 kJmol and the interionic distance is 269 pm. Thus point 2 addresses point 2 in your question K is bigger than Li. Web The lattice energy of KF is 794 kJmol. Medium View solution A sample of gas undergoes expansion against a pressure of 1 atm from a volume off 500mL to 900mL by adsorbing 500J of.
Web Lattice energy is a calculation of ionic bond strength in an ionic compound. Web Instead the lattice energy is calculated by subtracting the other four energies in the BornHaber cycle from the net enthalpy of formation. Web The Lattice energy U is the amount of energy required to separate a mole of the solid s into a gas g of its ions. The Na-F distance is 231 pm.
The formula that relates the lattice energy with charge and interionic. Web answer the questions below. What is lattice energy explain its. Also it can be described as a method of measuring cohesive forces that bind ions.
Because the ionic radii of the cations decrease. Web High charges on the ions mean high lattice energy. Redox Coordination Kf. Web lattice energy of M g O 3791 k J m o l Q.
A KFKClKBrKI B KIKBrKClKF C KFKClKIKBr D KIKBrKFKCl Hard Solution Verified by Toppr. The interionic distance of KF is 269 pm. A KFKClKBrKI B KIKBrKCLKF C KFKClKIBr D KIKBrKFKCl Medium Solution Verified by. Determine the lattice energy of KF s.
Web The lattice energies of KF KCl KBr and KI follow the order. LiF has a lattice energy of 1030. Estimating lattice energy using the Born-Haber cycle has been discussed in Ionic Solids. Web This problem has been solved.
Youll get a detailed solution from a subject matter expert that helps you learn core concepts. Web In one definition the lattice energy is the energy required to break apart an ionic solid and convert its component atoms into gaseous ions. Web The lattice energies of KF KCl KBr and KI follow the order. Given sublimation and ionization energy of Na are 108 kJmol and 496 kJmol respectively and bond dissociation energy required to.
Web 16 rows Lattice thermodynamics. Web Calculate the lattice energy of KF s using the following thermodynamic data all data is in kJmol. Web Figure 422 A Plot of Lattice Energy versus the Identity of the Halide for the Lithium Sodium and Potassium Halides. This definition causes the value.
Which of the following. Web The lattice energy decreases by a factor of 4. It gives insights into. Small separation means high lattice energy.
By comparing the lattices of substances from the same group or period the lattice energy trend may be determined. Use the data given to calculate an. KCI KBr and KI follow the order aKF KCl KrKI b KI KBr KClKF c KF KCl KI KBr d KI KBr KF KCl. Web How do you find the lattice energy trend.
1 KF 2 RbF 3 Neither they form equally strong attractions with water 4 it has a more negative heat of.
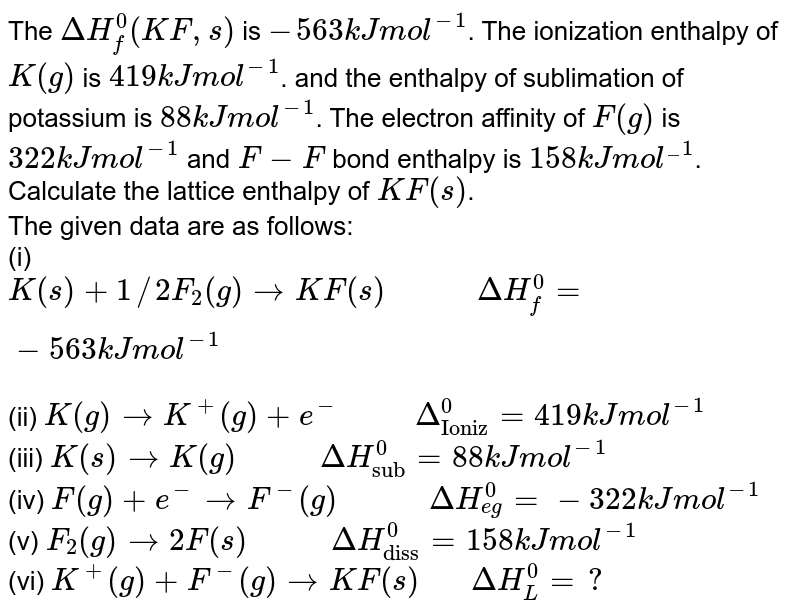 |
| The Deltah F 0 Kf S Is 563 Kj Mol 1 The Ionization Enthalpy Of K G Is 419 Kj Mol 1 And The Enthalpy Of Sublimation Of Potassium Is 88 Kj Mol 1 The Electron Affinity Of F G Is |
 |
| Ionic And Covalent Compounds Ppt Download |
 |
| Solved Use The Born Haber Cycle To Calculate The Lattice Chegg Com |
 |
| Solved Place The Following In Order Of Decreasing Magnitude Chegg Com |

Posting Komentar untuk "lattice energy of kf"